Tendons are sturdy bands of connective tissue that function by connecting muscles to bones. These channel the force created by each individual muscle to move the bone. Because of this, they must be sufficiently powerful to endure the force which is conducted through them yet sufficiently flexible to act as pulleys around bony prominences.
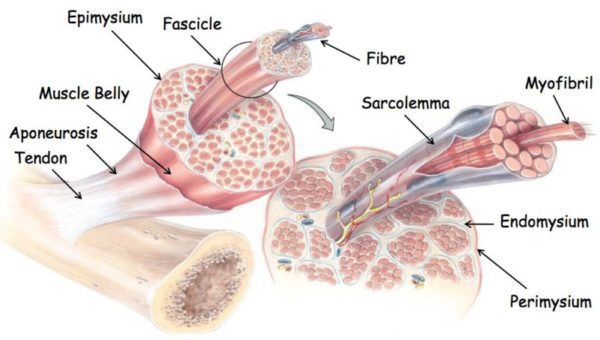
Collagen is a special protein, found in the extracellular matrix, or ECM, of connective tissue, which provides the tendons with enough tensile strength to allow them to stretch accordingly without tearing. The collagen molecules bind together to form a micro fibril, and multiple micro fibrils join into each other to form a collagen fibre. A group of collagen fibres forms a fibre bundle, and many fibre bundles joined together are called a tendon fascicle.
Each fibre bundles and tendon fascicles are enveloped in a thin layer of loose connective tissue medically referred to as the endotenon. The endotenon allows the bundles and fascicles to function independently of one another, sliding against one another according to each movement in order to properly adjust to the force and angle between the activity of the muscles and bones. The endotenon is also an addition of the connective tissue that encloses the entire tendon, called the epitenon. Some tendons have a supplementary sheath-like covering called the peratenon, which may function similarly alongside a tendon but it’s a distinct structure.
Within the tendon, there is a cell called the tenocyte that regulates and balances the secretion of the extracellular matrix and the accumulation of the collagen within the tendon. Tenocytes can be found in long rows along the collagen fibres and can also form in the endotenon and epitenon of the tendon. The tenocytes arrange into a connective web of finger-like extensions which allow the cells to communicate with each other according to the required needs for synthesis or degradation of collagen fibres.
These trigger the development of more collagen cells when they undergo stresses for short periods of time. However, tension for extended periods of time can cause collagen inhibition where the blood supply to tendons is considerably less than that of the muscle to which they are attached. The vessels that do exist within the tendon are particularly small, and run alongside the fascicles within the endotenon coat. Some areas of tendon lack a blood supply altogether. If such occurs, these areas can be especially vulnerable to degeneration and rupture.
Tendon Injury
Athletes who frequently overuse their muscles or as a result of direct trauma can develop a tendon injuries. Of the 32 million musculoskeletal injuries documented in the United States annually, 45% of them are injuries to tendons, ligaments, or joint capsules. The most common tendon injury types include the tendons of the rotator cuff of the shoulder, the Achilles tendon, patellar tendon, and the elbow extensor tendon. Several factors can place additional strain on the tendon and contribute to injuries caused by overuse, including: abnormal direction of pull due to skeletal misalignment; differences in limb lengths; muscle weakness or imbalances; hypermobile joints; inflexible muscles; training errors; and faulty or improperly fitted equipment and/or footwear.
Tendon injuries are believed to be difficult to treat as they were once historically thought of as an inflammatory condition, referred before to as tendonitis. Its treatment was therefore focused on reducing the inflammation through traditional anti-inflammatory medications and modalities and was extensively unsuccessful. Further research then demonstrated that acute inflammatory cells were missing, despite a disruption to the collagen composition within the injured tendon. A new term, tendinosis, was proclaimed to describe the degenerative injuries observed in the tendon tissue and the absence of inflammation. Regardless of the new classification and the inclusion of innovative therapies to address the degeneration of the tendon rather than the inflammation, successful treatment of tendon dysfunction, otherwise known as tendinopathy, has remained ambiguous.
The Significance of Inflammation
Medical advances now allow researchers to take a closer look at the process of tendinopathy. Studies of injured human tendon are difficult because by the time a person seeks medical help the injury is generally considered chronic. Therefore, animal models were studied to reveal acute tendon changes. Researchers from Queen Mary University in London evaluated the response of the tenocytes of horse tendons to cyclic loading. Fascicle bundles from six horses were divided into treatment and control groups for the study. Then, treatment samples sustained repeated loading strain, while controls remained unloaded.
After a 24-hour cyclic loading protocol, the collagen cells within the fascicles of the treated tendons appeared rounded and unorganized, meanwhile the control collagen cells were long, thin, and aligned longitudinally along the fascicle. Indications of inflammation were found in the treated samples after their loading cycle, while the control samples displayed only a few if any indications of inflammation. The scientists of the study ultimately concluded that tendon cells respond to increased levels of stress with an inflammatory reaction, especially in the acute period following injury.
These findings correspond with other animal studies which have found an increase in inflammatory indications after multiple circumstances of tendon damage as well as an increase in the number and size of the tenocytes. The increased production of tenocytes is known to occur in the presence of inflammation; therefore, this reaction believed to reveal a prior surge of inflammation. While degeneration has been diagnosed in chronic tendon injury, inflammation may cause those changes within the tendon during the acute period of tendon injury.
Further evidence
Once the injured tendons were examined with ultrasound, there appeared to be an increase in blood flow to the tendons. Healthy tendons are characteristically lacking in blood supply, therefore, to achieve this increased circulation, new blood vessels must penetrate the tendon. Known as neovascularization, this process generally occurs in conjunction within a nerve alongside the blood vessel. The development of new nerves within the injured tendon is thought to be the source of pain in tendinopathy.
The increase of blood flow is assumed to be evidence of degeneration within the tendon and an effort at healing the damaged tissue. Such neovascularization and neo innervation could likely not occur without the presence of inflammation. Researchers at Cambridge University show that the appearance of tendinopathy due to overload or injury can be characterized on ultrasound apart from those patients with known inflammatory conditions such as rheumatoid arthritis.
Biochemical influences
The enzyme cyclooxygenase-2, or COX-2, which in the presence of arachidonic acid, stimulates the production of prostanoids and produces inflammation. Studies show that levels of prostanoids are increased in animal tendons subjected to repeated loading. In tendons administered with injected prostanoids, the changes recorded within the tendon are consistent with tendinopathy. Therefore, the presence of greater levels of prostanoids in injured tendons can be ruled as clear evidence of an inflammatory process within the tendon.
Substance P is a peptide secreted by nerves and inflammatory cells. The presence of substance P in considerable amounts in chronic tendinopathy is thought to be the result of an inflammatory process within the tendon. Substance P causes an increase in the number of tenocytes within a tendon. As a result, the increased number of tenocytes discovered in an injured tendon may be the result of inflammatory mediators such as substance P. Substance P also increases the amount of collagen III to collagen I molecules in the extracellular matrix, or ECM. Within a healthy tendon, collagen I is the prevalent type found within the ECM. This change in the balance of the collagen may well explain the difference in the shape and size of the collagen and simultaneous disorganization observed in a study conducted in London.
Degeneration theory
Scientists in Melbourne, Australia, developed a multi-stage model of tendon injury that surrounds the current thinking on tendinosis. When a healthy tendon experiences increased amounts of weight, it responds by increasing its stiffness to handle the greater force demand and increases the production of collagen cells. The Australian researchers suggested that this non-inflammatory cell response, is a reactive tendinopathy. Their thought is that the increase in cells is an attempt by the tendon to increase the cross-sectional area and therefore, better handle the increased force on it. This short-term adaptation can be unpredictable if the added load is gradually decreased or the tendon has a chance to rest before the next amount of increased pressure is applied. A healthy tendon may easily adapt to the stress by growing larger and thus stronger, but a damaged or injured tendon does not recover from the stress and progresses to stage two.
In the second stage, identified as tendon disrepair, the tendon attempts to heal itself by adding more cells to the ECM, increasing the protein production of proteoglycans and collagen. It’s believed that these alter the composition and appearance of the collagen and gives the ECM a more disorganized appearance. According to the scientists, the composition of the ECM can be altered and healing may still take place at this stage.
The final stage is medically referred to as degenerative tendinopathy. The indication of this stage is cell death, with areas of tendon completely lacking healthy cells and an ECM filled with vessels and metabolic by-products, among several other things. This stage is considered irreversible. Degenerative tendinopathy is found as distinct lesions within a tendon. The injured tendon may display various stages of degeneration throughout the tendon.
Chiropractic and Athletic Performance
Two sides of the same coin
Previous research reveals that inflammatory alterations and degenerative alterations are both found within the same tendon. A group of researchers from Italy and Sweden suggested a different model for tendinopathy, around both the inflammatory and degenerative observations. Termed the “iceberg theory”, this model begins with the assumption that normal exercise may stimulate the production of new collagen within a tendon. Simultaneously, collagen degradation also occurs, most probably to reconstruct the tendon in order to accommodate the new amount of stress and pressure. Therefore, exercise stimulates the production of inflammation and growth substances, both which are needed for stimulating a healthy tendon. In healthy tendons, the tendon becomes larger and stronger.
When a tendon experiences constant strain or overload, the collagen fibres within the tendon begin to shift past one another, breaking their connective bonds. This micro-trauma is believed to weaken the tendon, and affects both the ECM and the blood supply. Vigorous or repeated exercise also increases the temperature within the tendon tissue. Decreasing the buildup of heat is difficult in tendons. The scientists theorize that it may be the hyperthermia within the tendon that causes the degeneration of cells rather than hypoxia.
Stopping any activity where weight is added to the region of the affected tendon, plenty of rest and adequate blood supply are needed for the tendon to heal from excessive strain. If the tendon does not have the required blood supply, factors are produced which stimulate angiogenesis. The appearance of new vessels, which typically include nerves alongside the blood vessels, is hypothesized to weaken the structure of the tendon. The secretion of both glutamate and substance P by the creation of nerves can contribute to a neurogenic inflammation as well as tendon cell death. It is at this point in the continuum, that athletes may complain of pain and seek medical attention.
Clinical relevance
Understanding that there may be both inflammation and degeneration involved in chronic tendinopathy may improve treatment outcomes. Since inflammation occurs early in the course of tendinopathy, non-steroidal anti-inflammatory drugs, or NSAIDS, and steroid injections may be most effective at the onset of pain or when the athlete suffers strain of the tendon. Sclerosing therapy and eccentric exercise both function to eliminate or reduce the number of new blood vessels and nerves in the tendon. By reducing the amount of new vessels, the tendon can return to normal and while reducing symptoms of pain. Eccentric exercise has an additional benefit of stimulating collagen production. Manual therapies, such as augmented soft tissue mobilization, are thought to also stimulate collagen production and return the ratio of type III collagen to type I collagen within the ECM to normal.
This new standard of tendon injuries and conditions creates new ideas for treatments. Treatments currently under investigation include biologic therapy, nitrous oxide, biochemical scaffolding, exogenous growth factor, platelet rich plasma injection, stem cell injection, and tissue engineering. Further research is needed to isolate which tendons and in what stage of injury, respond best to which therapy. In the meantime, the best recommendation is to treat any tendinopathy early when the anti-inflammatory methods are most effective and the chance for healing is greatest.
For more information, please feel free to ask Dr. Jimenez or contact us at 915-850-0900 . 
Sourced through Scoop.it from: www.elpasochiropractorblog.com
By Dr. Alex Jimenez
Post Disclaimer
Professional Scope of Practice *
The information herein on "Tendon Injury Caused by Physical Activity" is not intended to replace a one-on-one relationship with a qualified health care professional or licensed physician and is not medical advice. We encourage you to make healthcare decisions based on your research and partnership with a qualified healthcare professional.
Blog Information & Scope Discussions
Our information scope is limited to Chiropractic, musculoskeletal, physical medicines, wellness, contributing etiological viscerosomatic disturbances within clinical presentations, associated somatovisceral reflex clinical dynamics, subluxation complexes, sensitive health issues, and/or functional medicine articles, topics, and discussions.
We provide and present clinical collaboration with specialists from various disciplines. Each specialist is governed by their professional scope of practice and their jurisdiction of licensure. We use functional health & wellness protocols to treat and support care for the injuries or disorders of the musculoskeletal system.
Our videos, posts, topics, subjects, and insights cover clinical matters, issues, and topics that relate to and directly or indirectly support our clinical scope of practice.*
Our office has reasonably attempted to provide supportive citations and has identified the relevant research study or studies supporting our posts. We provide copies of supporting research studies available to regulatory boards and the public upon request.
We understand that we cover matters that require an additional explanation of how it may assist in a particular care plan or treatment protocol; therefore, to further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez, DC, or contact us at 915-850-0900.
We are here to help you and your family.
Blessings
Dr. Alex Jimenez DC, MSACP, RN*, CCST, IFMCP*, CIFM*, ATN*
email: coach@elpasofunctionalmedicine.com
Licensed as a Doctor of Chiropractic (DC) in Texas & New Mexico*
Texas DC License # TX5807, New Mexico DC License # NM-DC2182
Licensed as a Registered Nurse (RN*) in Florida
Florida License RN License # RN9617241 (Control No. 3558029)
License Compact Status: Multi-State License: Authorized to Practice in 40 States*
Presently Matriculated: ICHS: MSN* FNP (Family Nurse Practitioner Program)
Dr. Alex Jimenez DC, MSACP, RN* CIFM*, IFMCP*, ATN*, CCST
My Digital Business Card


