Epigenetic Abstract:
The increased prevalence of obesity and related comorbidities is a major public health problem. While genetic factors undoubtedly play a role in determining individual susceptibility to weight gain and obesity, the identified genetic variants only explain part of the variation. This has led to growing interest in understanding the potential role of epigenetics as a mediator of gene-environment interactions underlying the development of obesity and its associated comorbidities. Initial evidence in support of a role of epigenetics in obesity and type 2 diabetes mellitus (T2DM) was mainly provided by animal studies, which reported epigenetic changes in key metabolically important tissues following high-fat feeding and epigenetic differences between lean and obese animals and by human studies which showed epigenetic changes in obesity and T2DM candidate genes in obese/diabetic individuals. More recently, advances in epigenetic methodologies and the reduced cost of epigenome-wide association studies (EWAS) have led to a rapid expansion of studies in human populations. These studies have also reported epigenetic differences between obese/T2DM adults and healthy controls and epigenetic changes in association with nutritional, weight loss, and exercise interventions. There is also increasing evidence from both human and animal studies that the relationship between perinatal nutritional exposures and later risk of obesity and T2DM may be mediated by epigenetic changes in the offspring. The aim of this review is to summarize the most recent developments in this rapidly moving field, with a particular focus on human EWAS and studies investigating the impact of nutritional and lifestyle factors (both pre- and postnatal) on the epigenome and their relationship to metabolic health outcomes. The difficulties in distinguishing consequence from causality in these studies and the critical role of animal models for testing causal relationships and providing insight into underlying mechanisms are also addressed. In summary, the area of epigenetics and metabolic health has seen rapid developments in a short space of time. While the outcomes to date are promising, studies are ongoing, and the next decade promises to be a time of productive research into the complex interactions between the genome, epigenome, and environment as they relate to metabolic disease.
Keywords: Epigenetics, DNA methylation, Obesity, Type 2 diabetes, Developmental programming
Table of Contents
Introduction
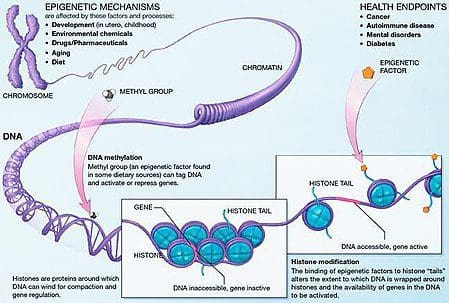 Obesity is a complex, multifactorial disease, and better understanding of the mechanisms underlying the interactions between lifestyle, environment, and genetics is critical for developing effective strategies for prevention and treatment [1].
Obesity is a complex, multifactorial disease, and better understanding of the mechanisms underlying the interactions between lifestyle, environment, and genetics is critical for developing effective strategies for prevention and treatment [1].
In a society where energy-dense food is plentiful and the need for physical activity is low, there is a wide variation in individuals’ susceptibility to develop obesity and metabolic health problems. Estimates of the role of heredity in this variation are in the range of 40–70 %, and while large genome-wide association studies (GWAS) have identified a number of genetic loci associated with obesity risk, the ~100 most common genetic variants only account for a few percent of variance in obesity [2, 3]. Genome-wide estimates are higher, accounting for ~20 % of the variation [3]; however, a large portion of the heritability remains unexplained.
Recently, attention has turned to investigating the role of epigenetic changes in the etiology of obesity. It has been argued that the epigenome may represent the mechanistic link between genetic variants and environmental factors in determining obesity risk and could help explain the “missing heritability.” The first human epigenetic studies were small and only investigated a limited number of loci. While this generally resulted in poor reproducibility, some of these early findings, for instance the relationship between PGC1A methylation and type 2 diabetes mellitus (T2DM) [4] and others as discussed in van Dijk et al. [5], have been replicated in later studies. Recent advances and increased affordability of high- throughput technologies now allow for large-scale epigenome wide association studies (EWAS) and integration of different layers of genomic information to explore the complex interactions between the genotype, epigenome, transcriptome, and the environment [6–9]. These studies are still in their infancy, but the results thus far have shown promise in helping to explain the variation in obesity susceptibility.
There is increasing evidence that obesity has develop mental origins, as exposure to a suboptimal nutrient supply before birth or in early infancy is associated with an increased risk of obesity and metabolic disease in later life [10–13]. Initially, animal studies demonstrated that a range of early life nutritional exposures, especially those experienced early in gestation, could induce epigenetic changes in key metabolic tissues of the offspring that persisted after birth and result in permanent alterations in gene function [13–17]. Evidence is emerging to support the existence of the same mechanism in humans. This has led to a search for epigenetic marks present early in life that predict later risk of metabolic disease, and studies to determine whether epigenetic programming of metabolic disease could be prevented or reversed in later life.
This review provides an update of our previous systematic review of studies on epigenetics and obesity in humans [5]. Our previous review showcased the promising outcomes of initial studies, including the first potential epigenetic marks for obesity that could be detected at birth (e.g., RXRA) [18]. However, it also highlighted the limited reproducibility of the findings and the lack of larger scale longitudinal investigations. The current review focuses on recent developments in this rapidly moving field and, in particular, on human EWAS and studies investigating the impact of (pre- and postnatal) nutritional and lifestyle factors on the epigenome and the emerging role of epigenetics in the pathology of obesity. We also address the difficulties in identifying causality in these studies and the importance of animal models in providing insight into mechanisms.
Review
Epigenetic Changes In Animal Models Of Obesity
 Animal models provide unique opportunities for highly controlled studies that provide mechanistic insight into the role of specific epigenetic marks, both as indicators of current metabolic status and as predictors of the future risk of obesity and metabolic disease. A particularly important aspect of animal studies is that they allow for the assessment of epigenetic changes within target tissues, including the liver and hypothalamus, which is much more difficult in humans. Moreover, the ability to harvest large quantities of fresh tissue makes it possible to assess multiple chromatin marks as well as DNA methylation. Some of these epigenetic modifications either alone or in combination may be responsive to environmental programming. In animal models, it is also possible to study multiple generations of offspring and thus enable differentiation between trans-generational and intergenerational transmission of obesity risk mediated by epigenetic memory of parental nutritional status, which cannot be easily distinguished in human studies. We use the former term for meiotic transmission of risk in the absence of continued exposure while the latter primarily entails direct transmission of risk through metabolic reprogramming of the fetus or gametes.
Animal models provide unique opportunities for highly controlled studies that provide mechanistic insight into the role of specific epigenetic marks, both as indicators of current metabolic status and as predictors of the future risk of obesity and metabolic disease. A particularly important aspect of animal studies is that they allow for the assessment of epigenetic changes within target tissues, including the liver and hypothalamus, which is much more difficult in humans. Moreover, the ability to harvest large quantities of fresh tissue makes it possible to assess multiple chromatin marks as well as DNA methylation. Some of these epigenetic modifications either alone or in combination may be responsive to environmental programming. In animal models, it is also possible to study multiple generations of offspring and thus enable differentiation between trans-generational and intergenerational transmission of obesity risk mediated by epigenetic memory of parental nutritional status, which cannot be easily distinguished in human studies. We use the former term for meiotic transmission of risk in the absence of continued exposure while the latter primarily entails direct transmission of risk through metabolic reprogramming of the fetus or gametes.
Animal studies have played a critical role in our current understanding of the role of epigenetics in the developmental origins of obesity and T2DM. Both increased and decreased maternal nutrition during pregnancy have been associated with increased fat deposition in offspring of most mammalian species studied to date (reviewed in [11, 13–15, 19]). Maternal nutrition during pregnancy not only has potential for direct effects on the fetus, it also may directly impact the developing oocytes of female fetuses and primordial germ cells of male fetuses and therefore could impact both the off- spring and grand-offspring. Hence, multigenerational data are usually required to differentiate between maternal intergenerational and trans-generational transmission mechanisms.
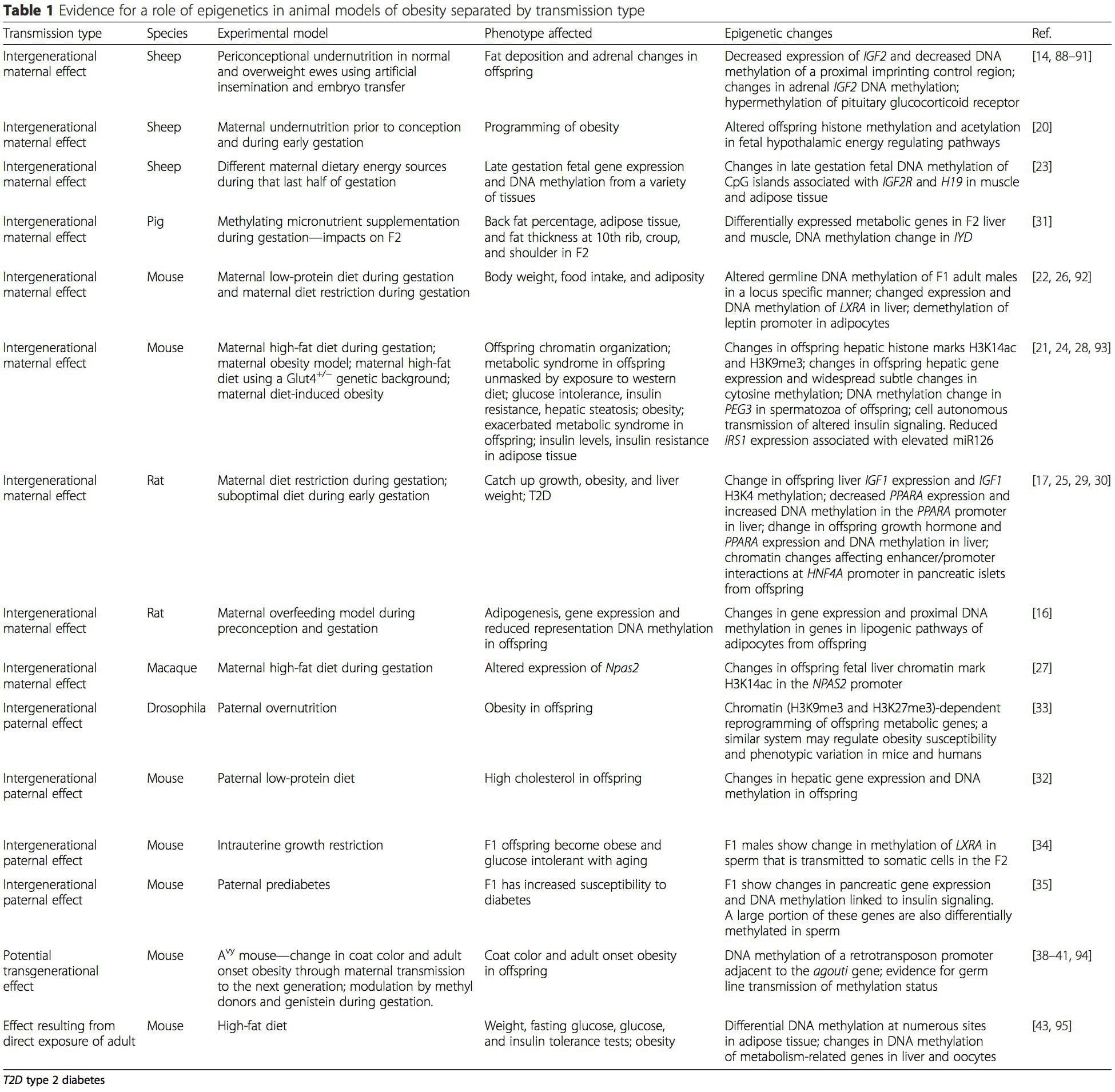
Table 1 summarizes a variety of animal models that have been used to provide evidence of metabolic and epigenetic changes in offspring associated with the parental plane of nutrition. It also contains information pertaining to studies identifying altered epigenetic marks in adult individuals who undergo direct nutritional challenges. The table is structured by suggested risk transmission type.
 (i) Epigenetic Changes In Offspring Associated With Maternal Nutrition During Gestation
(i) Epigenetic Changes In Offspring Associated With Maternal Nutrition During Gestation
 Maternal nutritional supplementation, undernutrition, and over nutrition during pregnancy can alter fat deposition and energy homeostasis in offspring [11, 13–15, 19]. Associated with these effects in the offspring are changes in DNA methylation, histone post-translational modifications, and gene expression for several target genes, especially genes regulating fatty acid metabolism and insulin signaling [16, 17, 20–30]. The diversity of animal models used in these studies and the common metabolic pathways impacted suggest an evolutionarily conserved adaptive response mediated by epigenetic modification. However, few of the specific identified genes and epigenetic changes have been cross-validated in related studies, and large-scale genome-wide investigations have typically not been applied. A major hindrance to comparison of these studies is the different develop mental windows subjected to nutritional challenge, which may cause considerably different outcomes. Proof that the epigenetic changes are causal rather than being associated with offspring phenotypic changes is also required. This will necessitate the identification of a parental nutritionally induced epigenetic “memory” response that precedes development of the altered phenotype in offspring.
Maternal nutritional supplementation, undernutrition, and over nutrition during pregnancy can alter fat deposition and energy homeostasis in offspring [11, 13–15, 19]. Associated with these effects in the offspring are changes in DNA methylation, histone post-translational modifications, and gene expression for several target genes, especially genes regulating fatty acid metabolism and insulin signaling [16, 17, 20–30]. The diversity of animal models used in these studies and the common metabolic pathways impacted suggest an evolutionarily conserved adaptive response mediated by epigenetic modification. However, few of the specific identified genes and epigenetic changes have been cross-validated in related studies, and large-scale genome-wide investigations have typically not been applied. A major hindrance to comparison of these studies is the different develop mental windows subjected to nutritional challenge, which may cause considerably different outcomes. Proof that the epigenetic changes are causal rather than being associated with offspring phenotypic changes is also required. This will necessitate the identification of a parental nutritionally induced epigenetic “memory” response that precedes development of the altered phenotype in offspring.
(ii)Effects Of Paternal Nutrition On Offspring Epigenetic Marks
 Emerging studies have demonstrated that paternal plane of nutrition can impact offspring fat deposition and epigenetic marks [31–34]. One recent investigation using mice has demonstrated that paternal pre-diabetes leads to increased susceptibility to diabetes in F1 offspring with associated changes in pancreatic gene expression and DNA methylation linked to insulin signaling [35]. Importantly, there was an overlap of these epigenetic changes in pancreatic islets and sperm suggesting germ line inheritance. However, most of these studies, although intriguing in their implications, are limited in the genomic scale of investigation and frequently show weak and somewhat transient epigenetic alterations associated with mild metabolic phenotypes in offspring.
Emerging studies have demonstrated that paternal plane of nutrition can impact offspring fat deposition and epigenetic marks [31–34]. One recent investigation using mice has demonstrated that paternal pre-diabetes leads to increased susceptibility to diabetes in F1 offspring with associated changes in pancreatic gene expression and DNA methylation linked to insulin signaling [35]. Importantly, there was an overlap of these epigenetic changes in pancreatic islets and sperm suggesting germ line inheritance. However, most of these studies, although intriguing in their implications, are limited in the genomic scale of investigation and frequently show weak and somewhat transient epigenetic alterations associated with mild metabolic phenotypes in offspring.
(iii)Potential Trans-generational Epigenetic Changes Promoting Fat Deposition In Offspring
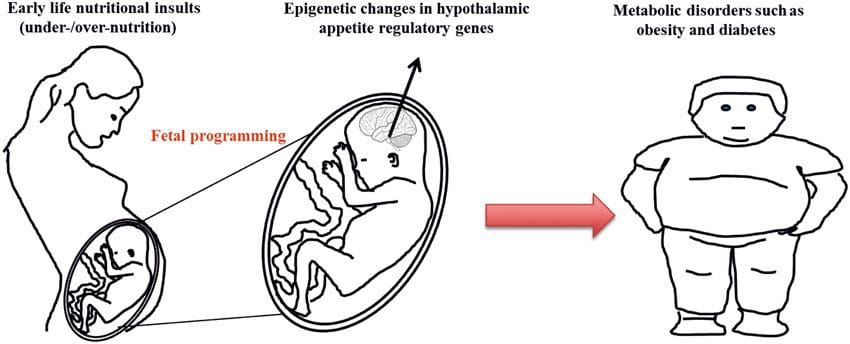 Stable transmission of epigenetic information across multiple generations is well described in plant systems and C. elegans, but its significance in mammals is still much debated [36, 37]. An epigenetic basis for grand- parental transmission of phenotypes in response to dietary exposures has been well established, including in livestock species [31]. The most influential studies demonstrating effects of epigenetic transmission impacting offspring phenotype have used the example of the viable yellow agouti (Avy) mouse [38]. In this mouse, an insertion of a retrotransposon upstream of the agouti gene causes its constitutive expression and consequent yellow coat color and adult onset obesity. Maternal transmission through the germ line results in DNA methylation mediated silencing of agouti expression resulting in wild-type coat color and lean phenotype of the offspring [39, 40]. Importantly, subsequent studies in these mice demonstrated that maternal exposure to methyl donors causes a shift in coat color [41]. One study has reported transmission of a phenotype to the F3 generation and alterations in expression of large number of genes in response to protein restriction in F0 [42]; however, alterations in expression were highly variable and a direct link to epigenetic changes was not identified in this system.
Stable transmission of epigenetic information across multiple generations is well described in plant systems and C. elegans, but its significance in mammals is still much debated [36, 37]. An epigenetic basis for grand- parental transmission of phenotypes in response to dietary exposures has been well established, including in livestock species [31]. The most influential studies demonstrating effects of epigenetic transmission impacting offspring phenotype have used the example of the viable yellow agouti (Avy) mouse [38]. In this mouse, an insertion of a retrotransposon upstream of the agouti gene causes its constitutive expression and consequent yellow coat color and adult onset obesity. Maternal transmission through the germ line results in DNA methylation mediated silencing of agouti expression resulting in wild-type coat color and lean phenotype of the offspring [39, 40]. Importantly, subsequent studies in these mice demonstrated that maternal exposure to methyl donors causes a shift in coat color [41]. One study has reported transmission of a phenotype to the F3 generation and alterations in expression of large number of genes in response to protein restriction in F0 [42]; however, alterations in expression were highly variable and a direct link to epigenetic changes was not identified in this system.
(iv) Direct Exposure Of Individuals To Excess Nutrition In Postnatal Life
 While many studies have identified diet-associated epigenetic changes in animal models using candidate site-specific regions, there have been few genome-wide analyses undertaken. A recent study focussed on determining the direct epigenetic impact of high-fat diets/ diet-induced obesity in adult mice using genome-wide gene expression and DNA methylation analyses [43]. This study identified 232 differentially methylated regions (DMRs) in adipocytes from control and high-fat fed mice. Importantly, the corresponding human regions for the murine DMRs were also differentially methylated in adipose tissue from a population of obese and lean humans, thereby highlighting the remarkable evolutionary conservation of these regions. This result emphasizes the likely importance of the identified DMRs in regulating energy homeostasis in mammals.
While many studies have identified diet-associated epigenetic changes in animal models using candidate site-specific regions, there have been few genome-wide analyses undertaken. A recent study focussed on determining the direct epigenetic impact of high-fat diets/ diet-induced obesity in adult mice using genome-wide gene expression and DNA methylation analyses [43]. This study identified 232 differentially methylated regions (DMRs) in adipocytes from control and high-fat fed mice. Importantly, the corresponding human regions for the murine DMRs were also differentially methylated in adipose tissue from a population of obese and lean humans, thereby highlighting the remarkable evolutionary conservation of these regions. This result emphasizes the likely importance of the identified DMRs in regulating energy homeostasis in mammals.
Human Studies
Drawing on the evidence from animal studies and with the increasing availability of affordable tools for genome- wide analysis, there has been a rapid expansion of epigenome studies in humans. These studies have mostly focused on the identification of site-specific differences in DNA methylation that are associated with metabolic phenotypes.
A key question is the extent to which epigenetic modifications contribute to the development of the metabolic phenotype, rather than simply being a con- sequence of it (Fig. 1). Epigenetic programming could contribute to obesity development, as well as playing a role in consequent risk of cardiovascular and metabolic problems. In human studies, it is difficult to prove causality [44], but inferences can be made from a number of lines of evidence:
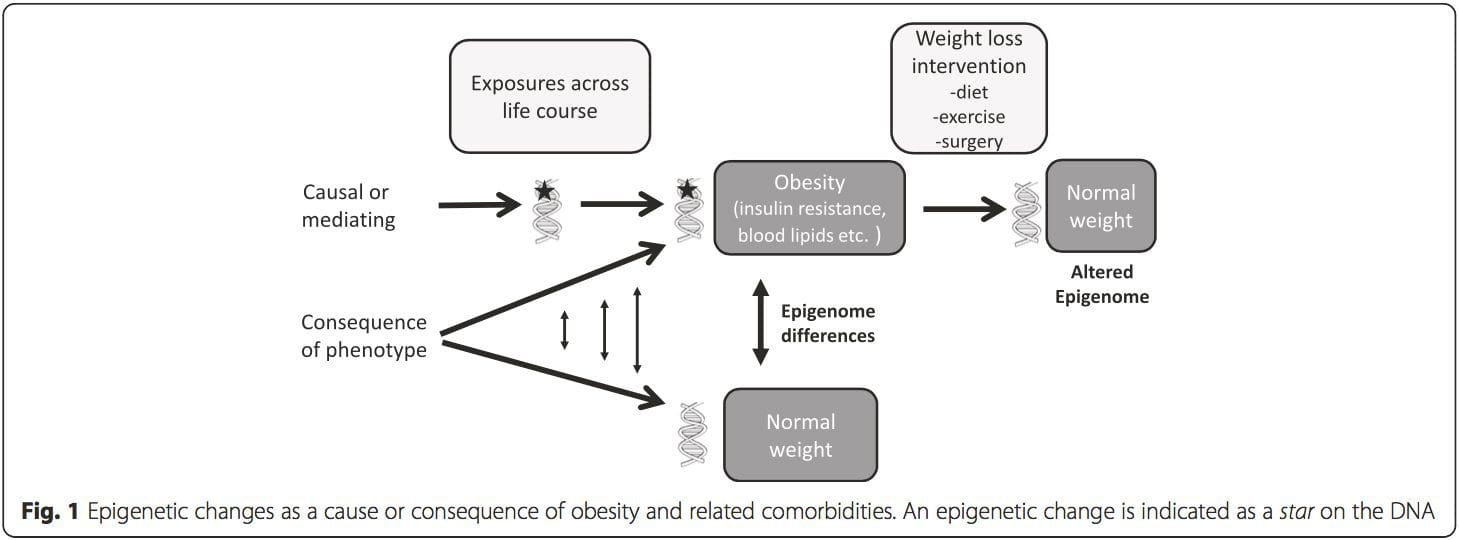 (i) Genetic association studies. Genetic polymorphisms that are associated with an increased risk of developing particular conditions are a priori linked to the causative genes. The presence of differential methylation in such regions infers functional relevance of these epigenetic changes in controlling expression of the proximal gene(s). There are strong cis-acting genetic effects underpinning much epigenetic variation [7, 45], and in population-based studies, methods that use genetic surrogates to infer a causal or mediating role of epigenome differences have been applied [7, 46–48]. The use of familial genetic information can also lead to the identification of potentially causative candidate regions showing phenotype-related differential methylation [49].
(i) Genetic association studies. Genetic polymorphisms that are associated with an increased risk of developing particular conditions are a priori linked to the causative genes. The presence of differential methylation in such regions infers functional relevance of these epigenetic changes in controlling expression of the proximal gene(s). There are strong cis-acting genetic effects underpinning much epigenetic variation [7, 45], and in population-based studies, methods that use genetic surrogates to infer a causal or mediating role of epigenome differences have been applied [7, 46–48]. The use of familial genetic information can also lead to the identification of potentially causative candidate regions showing phenotype-related differential methylation [49].
(ii)Timing of epigenetic changes. The presence of an epigenetic mark prior to development of a phenotype is an essential feature associated with causality. Conversely, the presence of a mark in association with obesity, but not before its development, can be used to exclude causality but would not exclude a possible role in subsequent obesity-related pathology.
(iii)Plausible inference of mechanism. This refers to epigenetic changes that are associated with altered expression of genes with an established role in regulating the phenotype of interest. One such example is the association of methylation at two CpG sites at the CPT1A gene with circulating triglyceride levels [50]. CPT1A encodes carnitine palmitoyltransferase 1A, an enzyme with a central role in fatty acid metabolism, and this is strongly indicative that differential methylation of this gene may be causally related to the alterations in plasma triglyceride concentrations.
Epigenome-Wide Association Studies: Identifying Epigenetic Biomarkers Of Metabolic Health
A number of recent investigations have focused on exploring associations between obesity/metabolic diseases and DNA methylation across the genome (Table 2). The largest published EWAS so far, including a total of 5465 individuals, identified 37 methylation sites in blood that were associated with body mass index (BMI), including sites in CPT1A, ABCG1, and SREBF1 [51]. Another large-scale study showed consistent associations between BMI and methylation in HIF3A in whole blood and adipose tissue [52], a finding which was also partially replicated in other studies [9, 51]. Other recently reported associations between obesity-related measures and DNA methylation include (i) DNA methylation differences between lean and obese individuals in LY86 in blood leukocytes [53]; (ii) associations between PGC1A promoter methylation in whole blood of children and adiposity 5 years later [54]; (iii) associations between waist-hip ratio and ADRB3 methylation in blood [55]; and (iv) associations between BMI, body fat distribution measures, and multiple DNA methylation sites in adipose tissue [9, 56]. EWAS have also shown associations between DNA methylation sites and blood lipids [55, 57–59], serum metabolites [60], insulin resistance [9, 61], and T2DM [48, 62, 63] (Table 2).
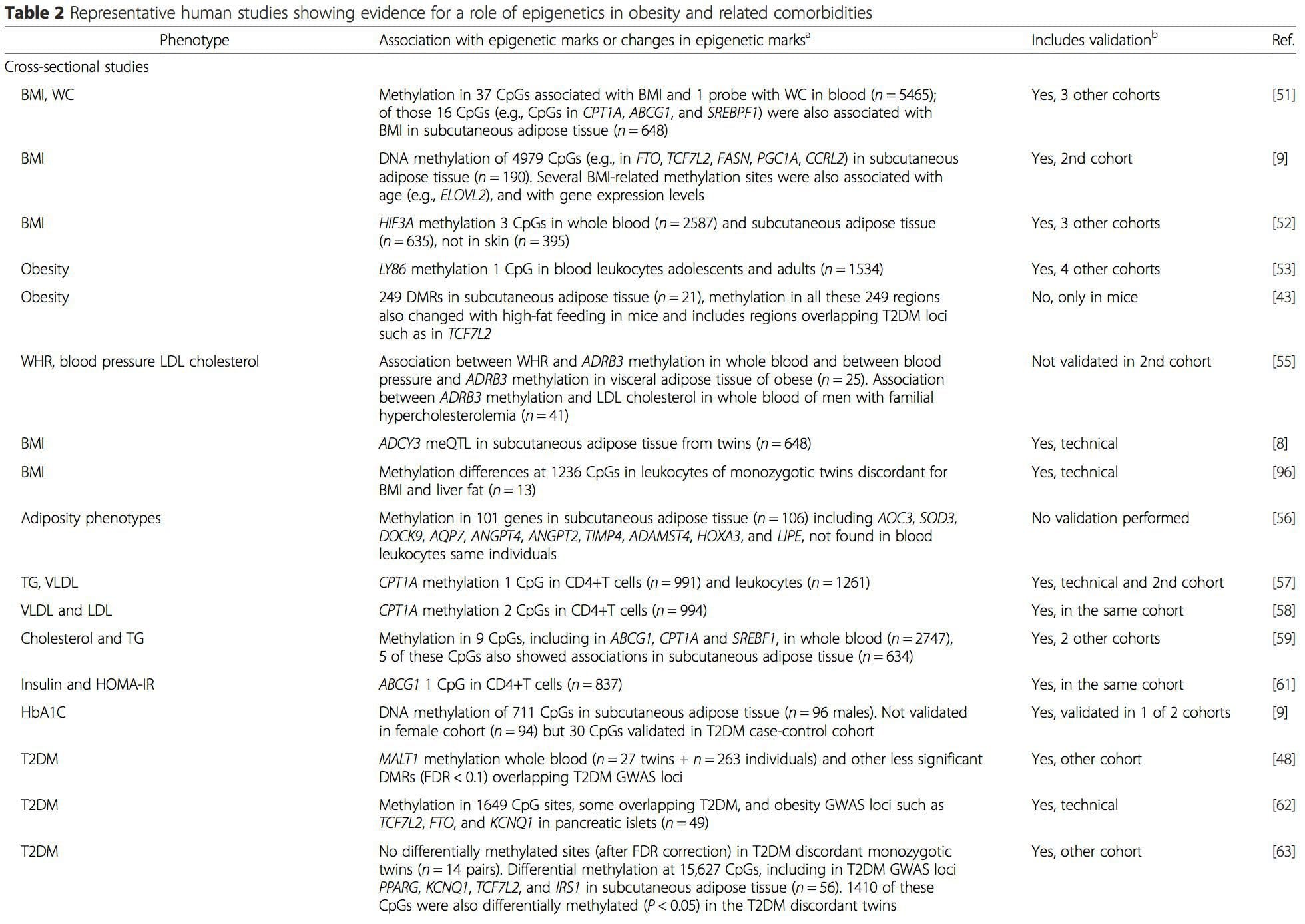
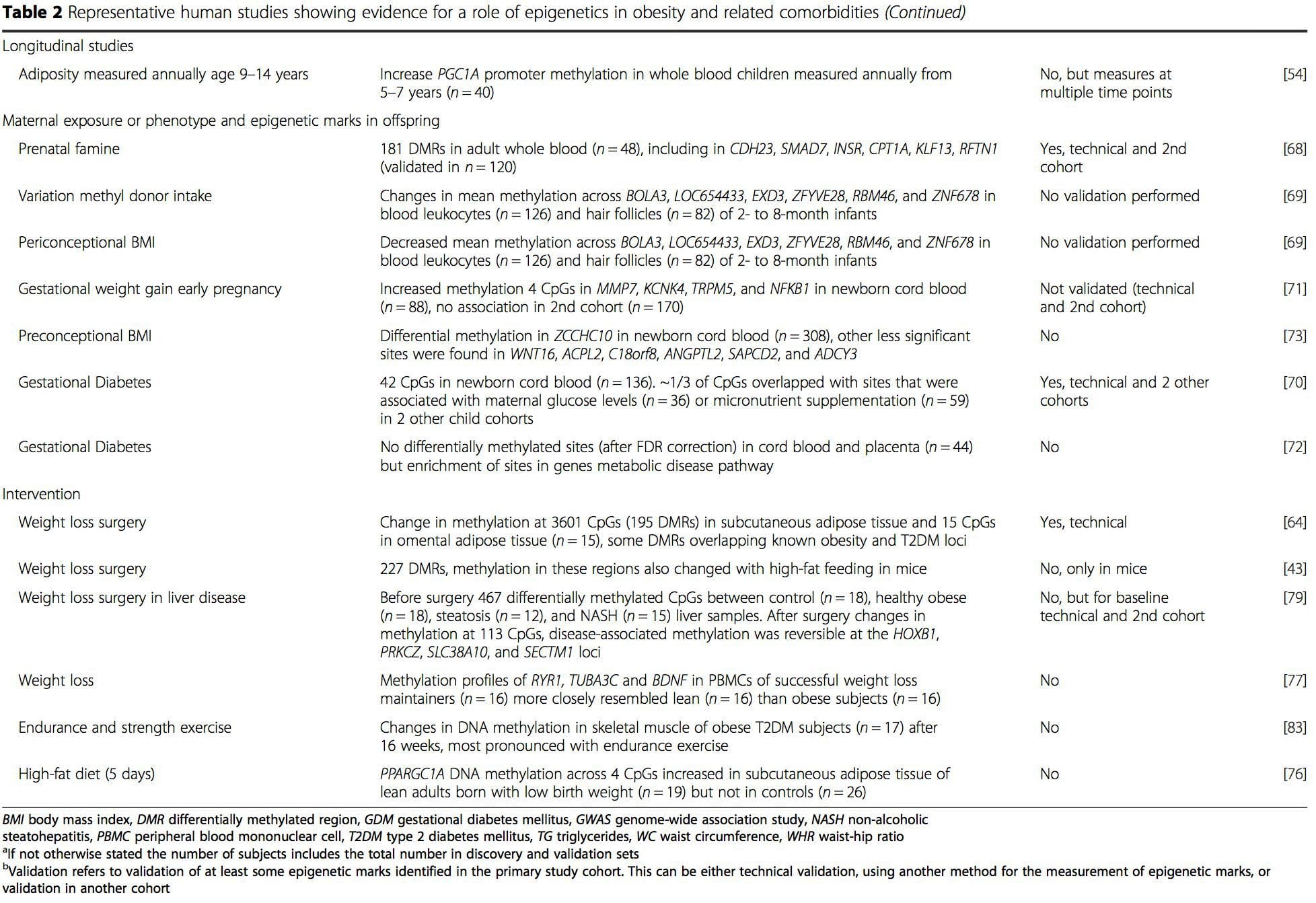 From these studies, altered methylation of PGC1A, HIF3A, ABCG1, and CPT1A and the previously described RXRA [18] have emerged as biomarkers associated with, or perhaps predictive of, metabolic health that are also plausible candidates for a role in development of metabolic disease.
From these studies, altered methylation of PGC1A, HIF3A, ABCG1, and CPT1A and the previously described RXRA [18] have emerged as biomarkers associated with, or perhaps predictive of, metabolic health that are also plausible candidates for a role in development of metabolic disease.
Interaction Between Genotype And The Epigenome
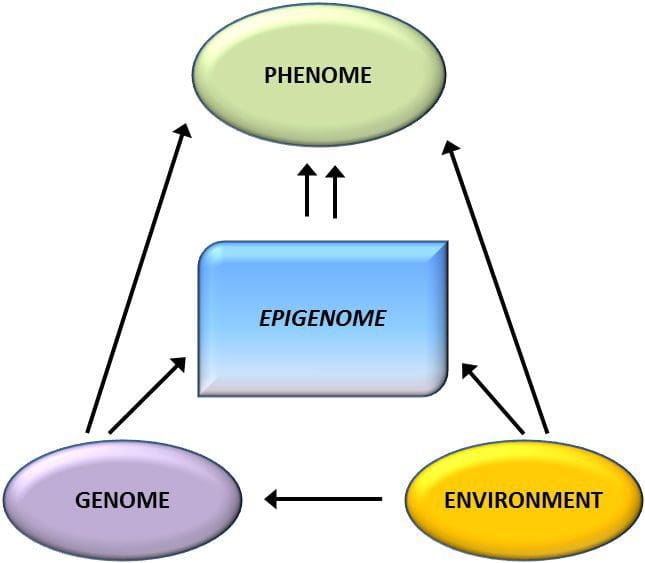 Epigenetic variation is highly influenced by the underlying genetic variation, with genotype estimated to explain ~20–40 % of the variation [6, 8]. Recently, a number of studies have begun to integrate methylome and genotype data to identify methylation quantitative trait loci (meQTL) associated with disease phenotypes. For instance, in adipose tissue, an meQTL overlapping with a BMI genetic risk locus has been identified in an enhancer element upstream of ADCY3 [8]. Other studies have also identified overlaps between known obesity and T2DM risk loci and DMRs associated with obesity and T2DM [43, 48, 62]. Methylation of a number of such DMRs was also modulated by high-fat feeding in mice [43] and weight loss in humans [64]. These results identify an intriguing link between genetic variations linked with disease susceptibility and their association with regions of the genome that undergo epigenetic modifications in response to nutritional challenges, implying a causal relationship. The close connection between genetic and epigenetic variation may signify their essential roles in generating individual variation [65, 66]. However, while these findings suggest that DNA methylation may be a mediator of genetic effects, it is also important to consider that both genetic and epigenetic processes could act independently on the same genes. Twin studies [8, 63, 67] can provide important insights and indicate that inter-individual differences in levels of DNA methylation arise predominantly from non-shared environment and stochastic influences, minimally from shared environmental effects, but also with a significant impact of genetic variation.
Epigenetic variation is highly influenced by the underlying genetic variation, with genotype estimated to explain ~20–40 % of the variation [6, 8]. Recently, a number of studies have begun to integrate methylome and genotype data to identify methylation quantitative trait loci (meQTL) associated with disease phenotypes. For instance, in adipose tissue, an meQTL overlapping with a BMI genetic risk locus has been identified in an enhancer element upstream of ADCY3 [8]. Other studies have also identified overlaps between known obesity and T2DM risk loci and DMRs associated with obesity and T2DM [43, 48, 62]. Methylation of a number of such DMRs was also modulated by high-fat feeding in mice [43] and weight loss in humans [64]. These results identify an intriguing link between genetic variations linked with disease susceptibility and their association with regions of the genome that undergo epigenetic modifications in response to nutritional challenges, implying a causal relationship. The close connection between genetic and epigenetic variation may signify their essential roles in generating individual variation [65, 66]. However, while these findings suggest that DNA methylation may be a mediator of genetic effects, it is also important to consider that both genetic and epigenetic processes could act independently on the same genes. Twin studies [8, 63, 67] can provide important insights and indicate that inter-individual differences in levels of DNA methylation arise predominantly from non-shared environment and stochastic influences, minimally from shared environmental effects, but also with a significant impact of genetic variation.
The Impact Of The Prenatal And Postnatal Environment On The Epigenome
 Prenatal environment: Two recently published studies made use of human populations that experienced “natural” variations in nutrient supply to study the impact of maternal nutrition before or during pregnancy on DNA methylation in the offspring [68, 69]. The first study used a Gambian mother-child cohort to show that both seasonal variations in maternal methyl donor intake during pregnancy and maternal pre-pregnancy BMI were associated with altered methylation in the infants [69]. The second study utilized adult offspring from the Dutch Hunger Winter cohort to investigate the effect of prenatal exposure to an acute period of severe maternal undernutrition on DNA methylation of genes involved in growth and metabolism in adulthood [68]. The results highlighted the importance of the timing of the exposure in its impact on the epigenome, since significant epigenetic effects were only identified in individuals exposed to famine during early gestation. Importantly, the epigenetic changes occurred in conjunction with increased BMI; however, it was not possible to establish in this study whether these changes were present earlier in life or a consequence of the higher BMI.
Prenatal environment: Two recently published studies made use of human populations that experienced “natural” variations in nutrient supply to study the impact of maternal nutrition before or during pregnancy on DNA methylation in the offspring [68, 69]. The first study used a Gambian mother-child cohort to show that both seasonal variations in maternal methyl donor intake during pregnancy and maternal pre-pregnancy BMI were associated with altered methylation in the infants [69]. The second study utilized adult offspring from the Dutch Hunger Winter cohort to investigate the effect of prenatal exposure to an acute period of severe maternal undernutrition on DNA methylation of genes involved in growth and metabolism in adulthood [68]. The results highlighted the importance of the timing of the exposure in its impact on the epigenome, since significant epigenetic effects were only identified in individuals exposed to famine during early gestation. Importantly, the epigenetic changes occurred in conjunction with increased BMI; however, it was not possible to establish in this study whether these changes were present earlier in life or a consequence of the higher BMI.
Other recent studies have provided evidence that prenatal over-nutrition and an obese or diabetic maternal environment are also associated with DNA methylation changes in genes related to embryonic development, growth, and metabolic disease in the offspring [70–73].
While human data are scarce, there are indications that paternal obesity can lead to altered methylation of imprinted genes in the newborn [74], an effect thought to be mediated via epigenetic changes acquired during spermatogenesis.
 Postnatal environment: The epigenome is established de novo during embryonic development, and therefore, the prenatal environment most likely has the most significant impact on the epigenome. However, it is now clear that changes do occur in the “mature” epigenome under the influence of a range of conditions, including aging, exposure to toxins, and dietary alterations. For example, changes in DNA methylation in numerous genes in skeletal muscle and PGC1A in adipose tissue have been demonstrated in response to a high-fat diet [75, 76]. Interventions to lose body fat mass have also been associated with changes in DNA methylation. Studies have reported that the DNA methylation profiles of adipose tissue [43, 64], peripheral blood mononuclear cells [77], and muscle tissue [78] in formerly obese patients become more similar to the profiles of lean subjects following weight loss. Weight loss surgery also partially reversed non-alcoholic fatty liver disease-associated methylation changes in liver [79] and in another study led to hypomethylation of multiple obesity candidate genes, with more pronounced effects in subcutaneous compared to omental (visceral) fat [64]. Accumulating evidence suggests that exercise interventions can also influence DNA methylation. Most of these studies have been conducted in lean individuals [80–82], but one exercise study in obese T2DM subjects also demonstrated changes in DNA methylation, including in genes involved in fatty acid and glucose transport [83]. Epigenetic changes also occur with aging, and recent data suggest a role of obesity in augmenting them [9, 84, 85]. Obesity accelerated the epigenetic age of liver tissue, but in contrast to the findings described above, this effect was not reversible after weight loss [84].
Postnatal environment: The epigenome is established de novo during embryonic development, and therefore, the prenatal environment most likely has the most significant impact on the epigenome. However, it is now clear that changes do occur in the “mature” epigenome under the influence of a range of conditions, including aging, exposure to toxins, and dietary alterations. For example, changes in DNA methylation in numerous genes in skeletal muscle and PGC1A in adipose tissue have been demonstrated in response to a high-fat diet [75, 76]. Interventions to lose body fat mass have also been associated with changes in DNA methylation. Studies have reported that the DNA methylation profiles of adipose tissue [43, 64], peripheral blood mononuclear cells [77], and muscle tissue [78] in formerly obese patients become more similar to the profiles of lean subjects following weight loss. Weight loss surgery also partially reversed non-alcoholic fatty liver disease-associated methylation changes in liver [79] and in another study led to hypomethylation of multiple obesity candidate genes, with more pronounced effects in subcutaneous compared to omental (visceral) fat [64]. Accumulating evidence suggests that exercise interventions can also influence DNA methylation. Most of these studies have been conducted in lean individuals [80–82], but one exercise study in obese T2DM subjects also demonstrated changes in DNA methylation, including in genes involved in fatty acid and glucose transport [83]. Epigenetic changes also occur with aging, and recent data suggest a role of obesity in augmenting them [9, 84, 85]. Obesity accelerated the epigenetic age of liver tissue, but in contrast to the findings described above, this effect was not reversible after weight loss [84].
Collectively, the evidence in support of the capacity to modulate the epigenome in adults suggests that there may be the potential to intervene in postnatal life to modulate or reverse adverse epigenetic programming.
Effect Sizes And Differences Between Tissue Types
![]() DNA methylation changes associated with obesity or induced by diet or lifestyle interventions and weight loss are generally modest (<15 %), although this varies depending on the phenotype and tissue studied. For instance, changes greater than 20 % have been reported in adipose tissue after weight loss [64] and associations between HIF3A methylation and BMI in adipose tissue were more pronounced than in blood [52].
DNA methylation changes associated with obesity or induced by diet or lifestyle interventions and weight loss are generally modest (<15 %), although this varies depending on the phenotype and tissue studied. For instance, changes greater than 20 % have been reported in adipose tissue after weight loss [64] and associations between HIF3A methylation and BMI in adipose tissue were more pronounced than in blood [52].
The biological relevance of relatively small methylation changes has been questioned. However, in tissues consisting of a mixture of cell types, a small change in DNA methylation may actually reflect a significant change in a specific cell fraction. Integration of epigenome data with transcriptome and other epigenetic data, such as histone modifications, is important, since small DNA methylation changes might reflect larger changes in chromatin structure and could be associated with broader changes in gene expression. The genomic context should also be considered; small changes within a regulatory element such as a promotor, enhancer, or insulator may have functional significance. In this regard, DMRs for obesity, as well as regions affected by prenatal famine exposure and meQTL for metabolic trait loci have been observed to overlap enhancer elements [8, 43, 68]. There is evidence that DNA methylation in famine-associated regions could indeed affect enhancer activity [68], supporting a role of nutrition-induced methylation changes in gene regulation.
A major limitation in many human studies is that epigenetic marks are often assessed in peripheral blood, rather than in metabolically relevant tissues (Fig. 2). The heterogeneity of blood is an issue, since different cell populations have distinct epigenetic signatures, but algorithms have been developed to estimate the cellular composition to overcome this problem [86]. Perhaps more importantly, epigenetic marks in blood cells may not necessarily report the status of the tissues of primary interest. Despite this, recent studies have provided clear evidence of a relationship between epigenetic marks in blood cells and BMI. In the case of HIF3A for which the level of methylation (beta-value) in the study population ranged from 0.14–0.52, a 10 % increase in methylation was associated with a BMI increase of 7.8 % [52]. Likewise, a 10 % difference in PGC1A methylation may predict up to 12 % difference in fat mass [54].
 Conclusions
Conclusions
The study of the role of epigenetics in obesity and metabolic disease has expanded rapidly in recent years, and evidence is accumulating of a link between epigenetic modifications and metabolic health outcomes in humans. Potential epigenetic biomarkers associated with obesity and metabolic health have also emerged from recent studies. The validation of epigenetic marks in multiple cohorts, the fact that several marks are found in genes with a plausible function in obesity and T2DM development, as well as the overlap of epigenetic marks with known obesity and T2DM genetic loci strengthens the evidence that these associations are real. Causality has so far been difficult to establish; however, regardless of whether the associations are causal, the identified epigenetic marks may still be relevant as biomarkers for obesity and metabolic disease risk.
Effect sizes in easily accessible tissues such as blood are small but do seem reproducible despite variation in ethnicity, tissue type, and analysis methods [51]. Also, even small DNA methylation changes may have biological significance. An integrative “omics” approach will be crucial in further unraveling the complex interactions between the epigenome, transcriptome, genome, and metabolic health. Longitudinal studies, ideally spanning multiple generations, are essential to establishing causal relationships. We can expect more such studies in the future, but this will take time.
While animal studies continue to demonstrate an effect of early life nutritional exposure on the epigenome and metabolic health of the offspring, human data are still limited. However, recent studies have provided clear evidence that exposure to suboptimal nutrition during specific periods of prenatal development is associated with methylation changes in the offspring and therefore have the potential to influence adult phenotype. Animal studies will be important to verify human findings in a more controlled setting, help determine whether the identified methylation changes have any impact on metabolic health, and unravel the mechanisms underlying this intergenerational/transgenerational epigenetic regulation. The identification of causal mechanisms underlying metabolic memory responses, the mode of transmission of the phenotypic effects into successive generations, the degree of impact and stability of the transmitted trait, and the identification of an overarching and unifying evolutionary context also remain important questions to be addressed. The latter is often encapsulated by the predictive adaptive response hypothesis, i.e., a response to a future anticipated environment that increases fi
Post Disclaimer
Professional Scope of Practice *
The information herein on "The Role Of Epigenetics In Obesity And Metabolic Disease" is not intended to replace a one-on-one relationship with a qualified health care professional or licensed physician and is not medical advice. We encourage you to make healthcare decisions based on your research and partnership with a qualified healthcare professional.
Blog Information & Scope Discussions
Our information scope is limited to Chiropractic, musculoskeletal, physical medicines, wellness, contributing etiological viscerosomatic disturbances within clinical presentations, associated somatovisceral reflex clinical dynamics, subluxation complexes, sensitive health issues, and/or functional medicine articles, topics, and discussions.
We provide and present clinical collaboration with specialists from various disciplines. Each specialist is governed by their professional scope of practice and their jurisdiction of licensure. We use functional health & wellness protocols to treat and support care for the injuries or disorders of the musculoskeletal system.
Our videos, posts, topics, subjects, and insights cover clinical matters, issues, and topics that relate to and directly or indirectly support our clinical scope of practice.*
Our office has reasonably attempted to provide supportive citations and has identified the relevant research study or studies supporting our posts. We provide copies of supporting research studies available to regulatory boards and the public upon request.
We understand that we cover matters that require an additional explanation of how it may assist in a particular care plan or treatment protocol; therefore, to further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez, DC, or contact us at 915-850-0900.
We are here to help you and your family.
Blessings
Dr. Alex Jimenez DC, MSACP, RN*, CCST, IFMCP*, CIFM*, ATN*
email: coach@elpasofunctionalmedicine.com
Licensed as a Doctor of Chiropractic (DC) in Texas & New Mexico*
Texas DC License # TX5807, New Mexico DC License # NM-DC2182
Licensed as a Registered Nurse (RN*) in Florida
Florida License RN License # RN9617241 (Control No. 3558029)
License Compact Status: Multi-State License: Authorized to Practice in 40 States*
Presently Matriculated: ICHS: MSN* FNP (Family Nurse Practitioner Program)
Dr. Alex Jimenez DC, MSACP, RN* CIFM*, IFMCP*, ATN*, CCST
My Digital Business Card



 (i) Epigenetic Changes In Offspring Associated With Maternal Nutrition During Gestation
(i) Epigenetic Changes In Offspring Associated With Maternal Nutrition During Gestation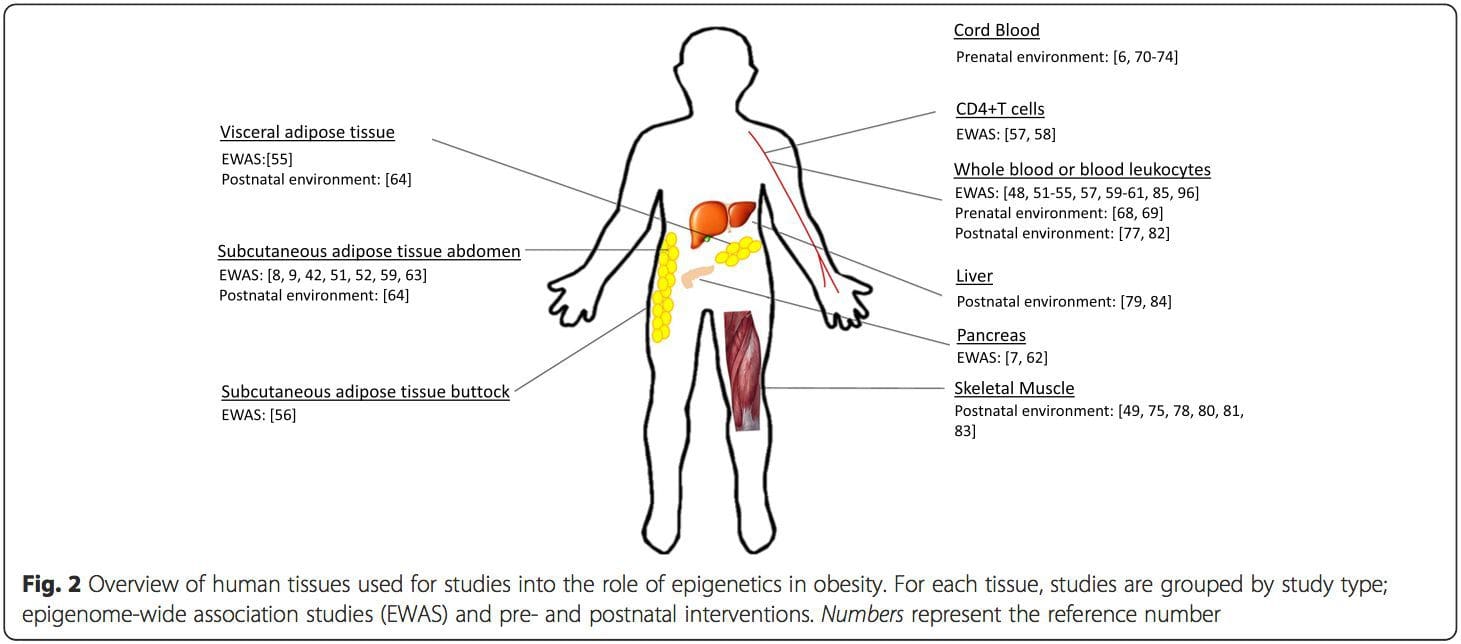 Conclusions
Conclusions